Neuroinflammation drives glaucoma pathology
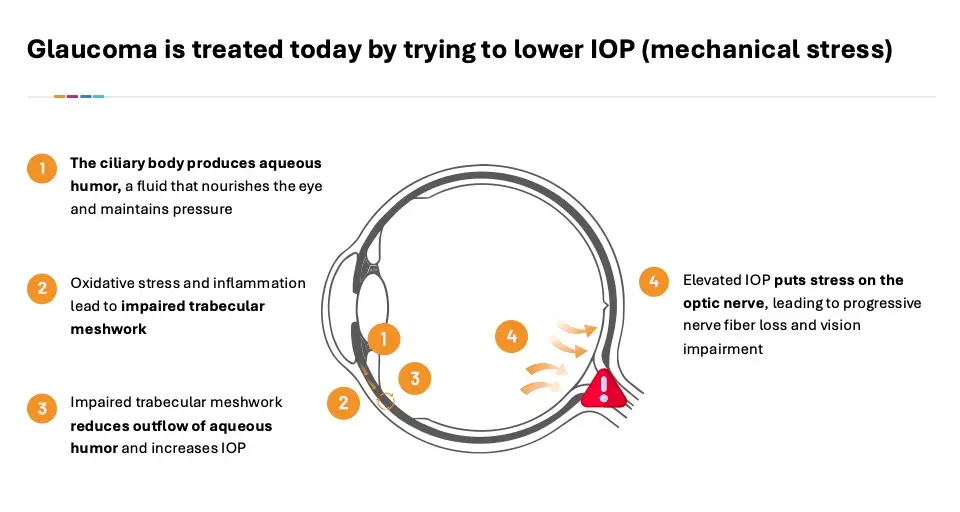
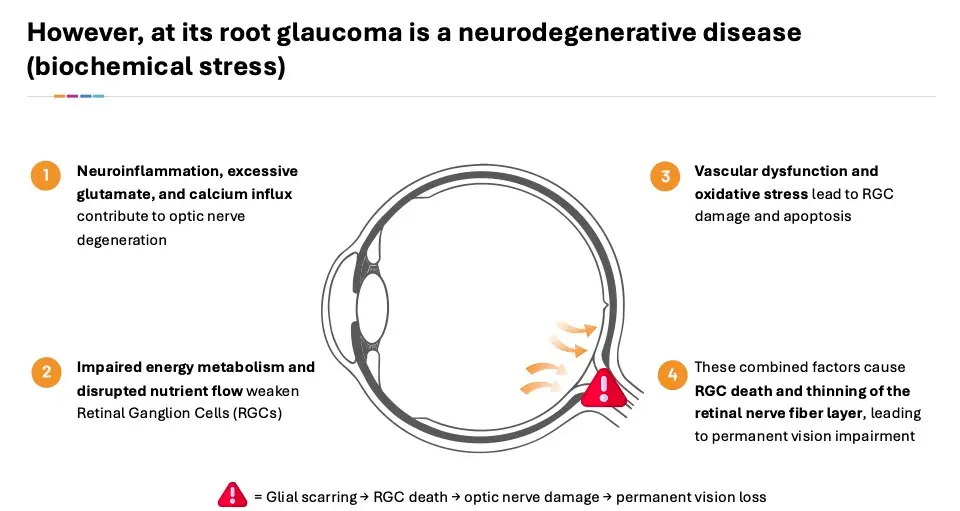
Glaucoma is the second leading cause of blindness globally. Glaucoma is caused by damage to the optic nerve in the back of the eye. Retinal ganglion cells (RGCs)—the nerve cells that send visual information from the eye to the brain—are damaged primarily through a combination of mechanical and biochemical stress.
Mechanically, increased pressure in the eye is caused by either elevated inflow or reduced outflow of aqueous humor in the front of the eye. This increased pressure compresses and distorts the axons of the RGCs, disrupting axonal transport. As a result, RGCs are starved of essential nutrients and signals, and they begin to die.
Biochemically, neuroinflammation contributes to the loss of RGCs. Supporting glial cells (like astrocytes and microglia) react to damage by releasing inflammatory cytokines and reactive oxygen species. While this is meant to protect, chronic inflammation worsens RGC death. In addition, excitotoxicity in the form of elevated levels of glutamate (a neurotransmitter) can become toxic to RGCs. Overactivation of glutamate receptors, especially NMDA receptors, causes calcium overload in the cell, leading to cell damage and death. Damage to mitochondria in RGCs may occur due to high metabolic demand and oxidative stress. This leads to the buildup of reactive oxygen species, damaging cellular components. This cascade of inflammation combines to starve the RGCs of nutrients and signals, resulting in cell death and loss of vision.
None of the topical therapies currently marketed address the fundamental biochemical issues that cause neuroinflammation.
About TAV-001
TAV-001 utilizes a dual mode of action that selectively targets the underlying mechanisms that cause inflammation and degeneration of the RGCs at the back of the eye, as well as IOP at the front of the eye. It targets a subunit of this protein which controls excitotoxicity and downstream inflammatory pathology, including damage to RGCs. In addition, it antagonizes a protein that controls both inflow and outflow of aqueous humor at the front of the eye.
In pre-clinical studies, TAV-001 has demonstrated signals of neuroprotection, including accumulation in the optic nerve and reduction in glial scarring, a hallmark of glaucoma progression. In addition, it has shown substantial, durable reduction in IOP, including against the current standard of care.
We expect to initiate a Phase 1 trial of TAV-001 in glaucoma patients in 2025.